View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Cheatgrass - Bromus tectorum
State Rank Reason (see State Rank above)
Bromus tectorum is native to southwestern Asia and was introduced into North America (Menalled et al. 2017). A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
Bromus tectorum is State Regulated, which means it is illegal to intentionally spread or sell.
General Description
PLANTS: An annual bunchgrass. Stems are erect, slender, and grow from 20–50 cm tall, depending upon soil moisture and plant density. Plants are green, but upon maturity turn red-brown. Stems are sparsely hairy (puberulent) below the inflorescence. Sources: Barkworth
in Flora of North America (FNA) 2007; Lesica et al. 2012.
LEAVES: Blades are 2–4 mm wide, up to 16cm long, and are softly hair on both sides. Sheaths are often densely and softly retrorsely pubescent to pilose, though sometimes the upper sheaths are glabrous. Ligules are membranous. Sources: Barkworth
in FNA 2007; Lesica et al. 2012.
INFLORESCENCE: An open, often nodding panicle of 2–15 cm long. Spikelets are 10–17 mm long with 3 to 6 florets. At maturity spikelets are red-brown to purple. Lemmas are 9–12 mm long, gradually tapered into two narrow teeth. The awn is straight or twisted, 12–20 mm long, and attached to the lemma. Sources: Barkworth
in FNA 2007; Lesica et al. 2012.
The common name “Downy Brome” refers to the numerous, fine hairs on its leaves.
The common name “Cheatgrass” comes from pioneering farmers who noticed it reduced their wheat yields, and in feeling cheated named it “cheatgrass”.
See
video on Identifying Invasive Annual Grasses in Montana.
Phenology
Cheatgrass is a winter annual (see REPRODUCTIVE CHARACTERISTICS section).
Diagnostic Characteristics
Many annual grasses can be confused with
Bromus tectorum. For the annual Bromes (
Bromus), Montana has 7 species
and only 3 are described below. A technical manual for identification is recommended, such as
Manual of Montana Vascular Plants (Lesica et al. 2022).
Cheatgrass –
Bromus tectorum, exotic, undesirable, and State-Regulated
* Seedlings have very hairy blades and sheaths.
* Mature plants turn reddish-purple.
* Awns are reddish-purple at maturity and easily stick to clothing and fur, and can get into the nostrils and eyes of animals.
* Glumes and lemmas are usually hairy. 1st Glume is 1-veined.
* Lemmas taper into 2 narrow teeth: bodies are 9-12 mm long and awns are greater than 10 mm.
Ripgut Brome -
Bromus diandrus, exotic and undesirable
* Lemmas taper into 2 narrow teeth: bodies are 20-35 mm long and awns are greater than 10 mm.
* 1st Glume is 1-veined.
Japanese Brome –
Bromus japonica, exotic and undesirable
* Plants tend to grow in more moist sites than does Cheatgrass (but can co-occur).
* Awns are straight or curved outward, less than 10 mm long.
* Glumes and awns not usually hairy
* 1st Glume is 3-to 5-veined.
* Spikelets often several from branch ends.
Ventenata –
Ventenata dubia, non-native, undesirable, Noxious
* Growth Form: Winter annual bunchgrass. Mature plants green to tawny brown.
* Leaves (mature): Narrow, folded lengthwise (inrolled), glabrous to puberulent,
and ascending. At the seedling stage, leaves are narrow and more needle-like when compared to Cheatgrass and Japanese Brome.
* Ligule: Long, membranous, 1-8 mm, and matched above by a purple-black node.
* Inflorescence & Stem: open, airy, pyramidal-shaped, upright panicle that emerges (May-June) while stems harden.
* Callus: Bearded (hairy)
* Glumes: 7-nerved
and smooth.
* Lemmas: Dimorphic. Lowest lemma with straight, persistent awn. Upper lemmas with a bifid tip and a twisted, bent awn (June-July), 1-1.6 cm long that disarticulates with the floret.
* Caryopsis: Seeds (from upper florets) retain bent, twisted awn after dispersal.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Cheatgrass is a plant from southwestern Asia (Mosley et al. in Sheley and Petroff 1999) and/or Europe (Barkworth in FNA 2007). It was likely introduced into North America several times (Mack 1981). Probably the earliest introductions came from contaminated soil used as ballast in ships coming from Eurasia (Mosley et al. in Sheley and Petroff 1999). Ballast dumped in St. Louis, Missouri about 1850 likely was contaminated with Cheatgrass (Mosley et al. in Sheley and Petroff 1999). In the western North America it was first reported in 1890 at Spences Bridge, British Columbia (Mosley et al. in Sheley and Petroff 1999). In 1892 it was found along the Northern Pacific Railroad near Ritzville, Washington. It was collected near Provo, UT in 1893, and reported in Nevada in 1906.
Cheatgrass dispersed in North America through transportation systems and the movement of goods (Mosley et al. in Sheley and Petroff 1999). Early infestations commonly occurred near wheat croplands where it contaminated wheat seed and straw. Contaminated straw used as packing material for merchandise transported by railroads. Straw used as bedding for livestock being moved via the railroad was often dumped within the rights-of-way. From these locations cheatgrass spread into native plant communities.
By 1914 Cheatgrass was widespread and locally abundant in the intermountain region (Mosley et al. in Sheley and Petroff 1999). By 1930 it has occupied most of its current range (Mosley et al. in Sheley and Petroff 1999). Cheatgrass presently occurs throughout most of North America (Lesica et al. 2012)
The earliest dated Montana specimens document Cheatgrass in 1898 in Missoula County, 1906 in Ravalli County, and 1914 in Gallatin County on the Montana State University campus (Posted by April 9, 2019 at http://www.pnwherbaria.org).
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
Observations in Montana Natural Heritage Program Database
Number of Observations: 13787
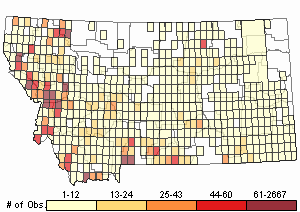
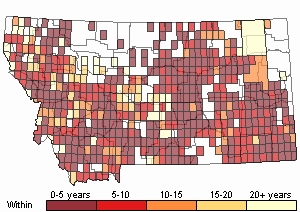
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
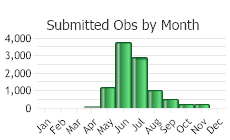
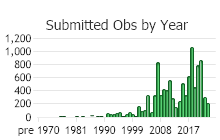
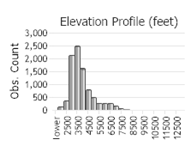 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Cheatgrass grows on overgrazed rangelands, fields, roadsides, waste places, sagebrush steppe, and open, dry understory (Lesica et al. 2012).
Ecology
DISPERSAL [Adapted from Mosley et al. in Sheley and Petroff 1999]
Cheatgrass has rapidly expanded into native plant communities throughout the intermountain west. This is partially because it was already pre-adapted to this climate. Following its introduction, the west experienced a period of excessive livestock grazing in the late 1800’s, which has reduced the competitiveness of native plants. Following World War I an agricultural depression occurred and many abandoned their dryland homesteads in the Great Basin (Piemeisel 1951). These abandoned cropland fields eventually colonized with Cheatgrass (Piemeisel 1951).
Cheatgrass can thrive in areas that have not been cultivated or grazed by domestic livestock. This is due to its reproductive strategies. Cheatgrass seeds are dispersed short-distances by the wind; its seeds being relatively large and heavy. Meanwhile, animals disperse its seeds long distances because they get caught in hair, feathers, clothing, vehicles and other mechanical equipment, mud attached to hooves and boots, and other vectors. If ingested seeds get transported in feces. Seeds lodged in hay and straw bales that remain dry in storage will remain viable for several years.
VULNERABLE HABITATS [Adapted from Mosley et al. in Sheley and Petroff 1999]
Cheatgrass is found from salt desert shrub communities that receive 15 cm of annual precipitation to high-elevation coniferous forests that receive at least 64 cm of annual precipitation. The most susceptible habitats have deep, loamy soils, south-facing slopes, and 12-22 inches of precipitation that predominantly falls in late winter to early spring.
Cheatgrass grows better in drier environments. It is usually a significant species in early-successional stages. It can be replaced in later-successional stages with perennial grasses. Cheatgrass is less competitive on well-managed foothill sites with perennial grasses. It usually is a minor species in later successional stages on moist sites.
In the drier prairie and steppe habitats, cheatgrass is very competitive and rapidly increases where perennial plants are stressed by drought, fire, or excessive grazing. Large seeded, annual grasses like Cheatgrass also colonize better where biological soils crusts are broken, partially disturbed or absent (Weber, Budel, and Belnap (editors) 2016). Cheatgrass tends to be inhibited where biological soils crusts are intact and healthy (Weber, Budel, and Belnap (editors) 2016; Serpe et al. 2006; Serpe et al. 2008). Colonization occurs in pulses. Cheatgrass populations can surge when growing conditions are favorable, then be unchanged for several years, until another pulse occurs. The critical point of expansion in Cheatgrass populations occurs when they influence the frequency of wildfires.
By changing the fire regime, Cheatgrass changes the composition of plant communities. This has become most noticeable in the sagebrush steppe. Prior to European settlement, intervals without fire lasted 20 to 25 years in the higher elevations of Mountain Big Sagebrush (Artemisia tridentata subspecies vaseyana) and 50 to 100 years in the drier Wyoming Big Sagebrush (Artemisia tridentata subspecies wyomingensis) in the Snake River Plain. However, current fire frequencies burn every 5 years or less in these cheatgrass-invaded habitats. The increased frequency in fire is due to their lifecycle that creates fuel for fires. Relative to perennial grasses, Cheatgrass plants remain green for 6-8 weeks or less. Upon maturity they become brown, dry, and their annual habit results in a higher accumulation of plant litter. Cheatgrass invaded habitats also burn more uniformly, leaving fewer unburned or lower severity burned areas.
The Cheatgrass-fire cycle is self-promoting (Pellant 1990; Peters and Bunting 1994). The native woody shrubs that occupy these habitats do not have reproductive strategies compatible to short-fire frequencies. Thus, winterfat (Krascheninnikovia lanata, Shadscale (Atriplex confertifolia), and Wyoming Big Sagebrush are removed from the system because they cannot survive and reproduce where fire is frequent. Even the more fire-tolerant, sprouting shrubs will eventually be lost because fires occur too frequently. Eventually, all perennial shrubs, grasses, and forbs are removed from the landscape. Cheatgrass establishment thrives because the shallow-rooted grasses more quickly absorb available moisture and nutrients, making it difficult for perennial native vegetation to establish or compete. In such areas where perennial plants area mostly absent and 5-year fire frequencies occur, the Cheatgrass community likely represents a stable community, self-perpetuating.
The annual life cycle of Cheatgrass adds organic matter to the soil surface, which acts as a mulch. This mulch favors the establishment of Cheatgrass seedlings while inhibiting the perennial bunchgrasses. Persistent and heavy mulch covers ground layer organisms, particularly biological soil crusts, eventually killing them (Weber, Budel, and Belnap (editors) 2016).
NUTRIONAL VALUE [Adapted from Mosley et al. in Sheley and Petroff 1999]
Cheatgrass plants are nutritive in the spring. The soft green leaves are palatable and nutritious with crude protein levels ranging from 15-18%. Livestock, including yearling ewes and steers, can gain weight on cheatgrass-dominated rangeland in the spring. Once Cheatgrass plant matures, weight gains drop to zero and plants become unpalatable because of the dry foliage and sharply-awned seeds. Livestock feeding on dry cheatgrass can get lumpy-jaw infections and severe eye injuries.
Cheatgrass florets are susceptible to Ergot, a fungus that is toxic to livestock.
COMPETITION [Adapted from Mosley et al. in Sheley and Petroff 1999]
Cheatgrass can out-compete other plants that are presumed to be competitive. Plants include: Halogeton (Halogeton glomeratus), Tall Tumble-mustard (Sisymbrium altissimum), and Russian Thistle (Salsola tragus). Cheatgrass outcompetes seedlings of many perennial plants because its shallow roots quickly capture available soil moisture. Competition from Cheatgrass can lead to failed seeding applications.
Reproductive Characteristics
Cheatgrass reproduces by seeds. Cheatgrass populations are genetically similar (Pyke and Novak 1994) but exhibit plasticity that allows it to survive under a variety of site conditions.
LIFE CYCLE [Adapted from Mosley et al. in Sheley and Petroff 1999]
Cheatgrass is an annual, completing its life cycle within one growing season. Typically, it grows, flowers, develops seeds, and becomes fully dry and dies within 2-3 months.
Specifically, Cheatgrass is usually a winter annual. Seeds typically germinate in the early fall when moisture becomes sufficient. They over-winter as small seedlings. However, if the climate is warm and rainy plants continue to grow. Sometimes when the above-growth is minimal the below-growth is more substantial. Cheatgrass roots can grow at cooler temperatures then many other plants can. In the spring plants resume growth when ambient temperatures warm. A single plant can consist of 1-20 tillers and its fibrous root system occurs within the upper 12 inches of the soil profile.
Plants are wind-pollinated. As soon as the fruits start to turn purple, but are largely still green, the seed is viable. Each Cheatgrass plant can produce a lot of seed. During unfavorable conditions, Cheatgrass may remain short, but will produce enough seed to perpetuate itself. Individual plants that grow in high densities can each produce 25 seeds. Individual plants that grow in lower densities with enough moisture and sunlight and that exhibit abundant tillers can produce 5,000 seeds.
Seeds on soil surface can germinate in mesic environments but need to be covered with soil or plant litter in drier climates. Upon sufficient moisture, up to 95% of Cheatgrass seeds will germinate and grow quickly.
Cheatgrass seeds can germinate in the spring, but plants are often fewer, less vigorous, and produce fewer seeds. Where spring is long and wet and there is abundant nitrification, many Cheatgrass seeds will lose their dormancy and germinate.
Management
Wherever Cheatgrass establishes it will be persistent, and eradication is not a reasonable goal in most situations. The strategy chosen to suppress Cheatgrass will determine its level of abundance and role in the community. Sites need to be evaluated to determine how much of the community still has perennial shrubs, forbs, and grasses. In sites devoid of perennial plants, the Cheatgrass community is likely stable and self-perpetuating and control techniques do not need to consider impacts to perennial plants. In sites with seral plant communities that have Cheatgrass, but also have perennial shrubs, forbs, and/or grasses, control techniques that do not harm the perennial vegetation should be implemented.
An integrated vegetative management approach provides the best long-term control, and requires that land-use objectives and a desired plant community be identified (Shelly et al.
in Sheley and Petroff 1999). Once identified an integrated weed management strategy to promote a weed-resistant plant community and serve other land-use objectives such as livestock forage, wildlife habitat, or recreation, can be developed.
PREVENTION [Adapted from Sheley and Petroff 1999]
Preventing the establishment of Cheatgrass can be accomplished by many practices:
* Learn how to accurately identify Cheatgrass in order to detect occurrences and know where to implement control methods.
* Implement measures to reduce soil disturbance and improve conditions for perennial plant reproduction.
* Prevent vehicles from driving through and animals from grazing within infested areas.
* Thoroughly wash the undercarriage of vehicles and wheels in a designated area before moving to uninfested areas.
* Frequently monitor for new plants, and when found implement effective control methods.
* Maintain proper grazing management that creates resilience to noxious weed invasion.
PHYSICAL and CULTURAL CONTROLS [Adapted from Mosley
in Sheley and Petroff 1999]
Hand-pulling is effective and easy in small areas, gardens, alleys, and other places, particularly when soils are moist. The shallow, fibrous roots system makes it easier for the entire plant to be pulled and bagged. If plants are in the fruit/seed stage then plants should be allowed to desiccate within sealed bags before depositing in the landfill. Hand-pulling should be done at intervals until the seed source is depleted.
Disking creates favorable soil conditions, stimulates seed germination, and is usually not effective. To be effective, tillage must be 10-15 cm deep in order to bury seeds and prevent their germination and be repeated during the season. Increasing the soil bulk density of the soil will inhibit Cheatgrass, but should not be done if it will impact desirable plants.
Revegetating land with competitive, locally adapted, and competitive grasses, forbs, and shrubs will develop a plant community that is more resilient to Cheatgrass. However, establishing the community can be difficult because cheatgrass plants quickly absorb available soil moisture and nutrients. It is necessary to use revegetation in combination with disking, herbicides, or prescribed fire on rangeland to be more effective in reducing Cheatgrass. Details at combining techniques can be found in Mosley
in Sheley and Petroff 1999 and with your local County Weed or Farm agents.
NOTE: Cultivars of native grasses do not break down and degrade like true native plants, and will contribute to fuel loads and harming biological soil crusts.
Prescribed Burning is not an effective control method. Burning will decrease above ground biomass, but stimulates seed germination, and decreases competition from desirable plants. Cheatgrass plants that establish after a fire produce more seed per plant.
Mowing is not an effective control method because it distributes seeds, decreases competition from adjacent plants, and maintains conditions that are favorable to Cheatgrass.
CHEMICAL CONTROLS [Adapted from Mosley
in Sheley and Petroff 1999]
Herbicides can be effective, when desired perennial plants are still abundant in the community. A single year of herbicide application will temporarily reduce the population, but increase seed production. Chemical control must be repeated for 2 to 5 consecutive years. Combining revegetation with herbicide control can be more effective if done properly. Details at combining techniques can be found in Mosley
in Sheley and Petroff 1999.
The herbicide type and concentration, application time and method, environmental constraints, land use practices, local regulations, and other factors will determine its effectiveness and impact to non-target species. Strict adherence to application requirements defined on the herbicide label will reduce risks to human and environmental health. Consult your County Extension Agent and/or Weed District for information on herbicidal control. Chemical information is also available at
Greenbook.
Paraquat (0.5 pounds active ingredient (ai) per acre) applied in the spring to the vegetative stage or early dough stage of seed development will provide control if repeated for 2 consecutive years. A surfactant will increase the effectiveness of paraquat.
Glyphosate (0.6 pounds ai per acre) applied in the spring will kill desirable perennial grasses. For Cheatgrass glyphosate (0.5 pounds ai per acre) applied in the spring to the vegetative stage or early dough stage of seed development will provide control if repeated for 3 consecutive years. For Cheatgrass glyphosate (0.4 pounds ai per acre) applied in the spring to the early dough stage of seed development will provide control if repeated for 3 consecutive years.
The active ingredient of indaziflam was previously marketed under the trade mark name of Esplanade which is restricted to industrial vegetation management. Indaziflam, under the trade mark name of Rejuvra has been approved by the Environmental Protection Agency (EPA) for use on rangeland, CRP, and natural areas including sites grazed by livestock. Rejuvra has successfully controlled annual grasses including Ventenata, Cheatgrass, Japanese Brome, and Medusahead. The chemical should be applied prior to seed germination; it interferes with cellulose biosynthesis to reduce emergence. For further research information, search the
Montana State University Extension and
University of Wyoming Weed Program websites.
BIOLOGICAL CONTROLS [Adapted from Mosley
in Sheley and Petroff 1999]
No biological control agents are available. Cheatgrass seedheads are susceptible to Ergot, a fungus which will kill seeds.
GRAZING CONTROLS [Adapted from Mosley
in Sheley and Petroff 1999]
Livestock grazing can be purposely designed to control Cheatgrass (Megee 1938; Daubenmire 1940; Valentine and Stevens 1994; and Mosely 1996). As a tool it works best when targeted to local areas for the purpose of protecting existing perennial plants from fire or aiding seeding restoration in severely depleted sites.
To control Cheatgrass targeted grazing must be done at least twice per season for at least two consecutive years. The grazing intensity should be light enough to maintain a minimum 8 cm (3 inches) stubble height on desirable grasses. The first grazing should be done in the spring when plants are tall enough to become accessible and palatable, but before plants turn purplish (before they reach the soft dough stage of seed development). This will prevent most seed development. The second grazing should occur in late spring during the boot stage, then allowed to re-grow for 3-4 weeks before re-grazing.
Grazing intensities in winter can be moderate to heavy without damaging perennial plants, as long as soils are dry and firm.
Prescribed grazing can also reduce litter build-up, disrupting the fire cycle, reducing the fuel loads, and enhance the competitiveness from perennial plants. Grazed firelines should be at least 75 meters (250 feet) wide.
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
Threats or Limiting Factors
Cheatgrass can significantly alter the composition of native rangeland vegetation because it is a strong colonizer and competitor and facilitates wildfires (Mosley et al. in Sheley and Petroff 1999). In drier prairies and sagebrush steppe habitats it has colonized ground that naturally had low frequencies of forbs and grasses and higher coverage of intact biological soils crusts. Where the soil surface becomes disturbed, cheatgrass plants easily colonize and develop dense populations. Their foliage dries out sooner than perennial grasses and their litter builds up creating more frequent fire intervals and more uniform fires (Mosley et al. in Sheley and Petroff 1999) See ECOLOGY / VULNERABLE HABITATS section.
Cheatgrass adds organic matter to the soil surface, which acts as a mulch. This mulch favors the establishment of Cheatgrass seedlings while inhibiting the perennial bunchgrasses. Persistent and heavy mulch covers ground layer organisms, particularly biological soil crusts, eventually killing them (Weber, Budel, and Belnap (editors) 2016).
Cheatgrass-infested rangelands have less plant production than native rangelands (Mosley et al. in Sheley and Petroff 1999). Cheatgrass plants are only nutritious when green and young, which lasts for about 6-8 weeks. Mostly perennial grasses remain green and nutritious for longer periods. The short ‘green’ period of Cheatgrass reduces the length of forage production that a site could support for livestock and native ungulates if occupied by perennial grasses.
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Daubenmire, R.F. 1940. Plant succession due to overgrazing in the Agropyron bunchgrass prairie of southeastern Washington. Ecology 21(1):55-64.
Daubenmire, R.F. 1940. Plant succession due to overgrazing in the Agropyron bunchgrass prairie of southeastern Washington. Ecology 21(1):55-64. Flora of North America Editorial Committee, eds. 2007. Flora of North America North of Mexico. Volume 24. Magnoliophyta: Commelinidae, Part 1. Oxford University Press, Inc., NY. xxxiii + 911 pp.
Flora of North America Editorial Committee, eds. 2007. Flora of North America North of Mexico. Volume 24. Magnoliophyta: Commelinidae, Part 1. Oxford University Press, Inc., NY. xxxiii + 911 pp. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Megee, C.R. 1938. Wild oats or downy brome. Mich. Agr. Exp. Sta. Occas. Bull. 20(3): 153-156.
Megee, C.R. 1938. Wild oats or downy brome. Mich. Agr. Exp. Sta. Occas. Bull. 20(3): 153-156. Mosley, G., R.S. Nowak, and R.J. Tausch. 1996. Prescribed sheep grazing to suppress cheatgrass: A review. Sheep and Goat Res. J. 12:74-81.
Mosley, G., R.S. Nowak, and R.J. Tausch. 1996. Prescribed sheep grazing to suppress cheatgrass: A review. Sheep and Goat Res. J. 12:74-81. Pellant, M. 1990. The cheatgrass-wildfire cycle - are there any solutions? In: E.D. McArthur, E. M Romney, S.D. Smith, and P.T. Tueller (eds.), Proc. Symp. on cheatgrass invasion, shrub die-off, and other aspects of shrub biology and management. USDA For. Ser. Gen. Tech. Rep. INT-276, 11-18.
Pellant, M. 1990. The cheatgrass-wildfire cycle - are there any solutions? In: E.D. McArthur, E. M Romney, S.D. Smith, and P.T. Tueller (eds.), Proc. Symp. on cheatgrass invasion, shrub die-off, and other aspects of shrub biology and management. USDA For. Ser. Gen. Tech. Rep. INT-276, 11-18. Peters, E.F., and S.C. Bunting. 1994. Fire Conditions pre- and post-occurrence of annual grasses on the Snake River Plain. In: S.B. Monsen and S.G. Kitchen (eds.), Proc. Ecology and management of annual rangelands. USDA For. Ser. Gen. Tech. Rep. INT-GRR-313, 31-36.
Peters, E.F., and S.C. Bunting. 1994. Fire Conditions pre- and post-occurrence of annual grasses on the Snake River Plain. In: S.B. Monsen and S.G. Kitchen (eds.), Proc. Ecology and management of annual rangelands. USDA For. Ser. Gen. Tech. Rep. INT-GRR-313, 31-36. Piemeisel, R.L. 1951. Causes affecting change and rate of change in a vegetation of annuals in Idaho. Ecology 32:53-72.
Piemeisel, R.L. 1951. Causes affecting change and rate of change in a vegetation of annuals in Idaho. Ecology 32:53-72. Serpe, MD, JM Orm, TR Barkes, R Rosentreter. 2006. Germination and seed water status of four grasses on moss dominated biological soil crusts from arid lands. Plant Ecol. 185: 163-178.
Serpe, MD, JM Orm, TR Barkes, R Rosentreter. 2006. Germination and seed water status of four grasses on moss dominated biological soil crusts from arid lands. Plant Ecol. 185: 163-178. Serpe, MD, SJ Zimmerman, L Deines, R Rosentreter. 2008. Seed water status and root tip characteristics of two annual grasses on lichen-dominated biological soil crusts. Plant Soil 303: 191-205.
Serpe, MD, SJ Zimmerman, L Deines, R Rosentreter. 2008. Seed water status and root tip characteristics of two annual grasses on lichen-dominated biological soil crusts. Plant Soil 303: 191-205. Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon. Vallentine, J. F. and A. R. Stevens. 1994. Use of livestock to control cheatgrass-a review. Pp. 202-210 in S. B. Monsen and S. G. Kitchen, eds. Proceedings: Ecology and management of annual rangelands. General Technical Report INT-GTR-313. Ogden, UT: Intermountain Research Station, Forest Service, U.S. Department of Agriculture.
Vallentine, J. F. and A. R. Stevens. 1994. Use of livestock to control cheatgrass-a review. Pp. 202-210 in S. B. Monsen and S. G. Kitchen, eds. Proceedings: Ecology and management of annual rangelands. General Technical Report INT-GTR-313. Ogden, UT: Intermountain Research Station, Forest Service, U.S. Department of Agriculture. Weber, Bettina, Burkhard Budel, and Jayne Belnap (editors). 2016. Biological Soil Crusts: An Organizing Principle in Drylands. Ecological Studies, Analysis and Synthesis, Volume 226. Springer International Publishing, Switzerland.
Weber, Bettina, Burkhard Budel, and Jayne Belnap (editors). 2016. Biological Soil Crusts: An Organizing Principle in Drylands. Ecological Studies, Analysis and Synthesis, Volume 226. Springer International Publishing, Switzerland.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Anderson, N.L. 1951. Field studies on the biology of range grasshoppers of southeastern Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 96 p.
Anderson, N.L. 1951. Field studies on the biology of range grasshoppers of southeastern Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 96 p. Aradottir, A.L. 1984. Ammonia volatilization from native grasslands and forests of SW Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 48 p.
Aradottir, A.L. 1984. Ammonia volatilization from native grasslands and forests of SW Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 48 p. Bachen, D.A. 2014. Cheatgrass invasion of sagebrush steppe: Impacts of vegetation structure on small mammals. M.Sc. Thesis. Bozeman, Montana: Montana State University. 96 p.
Bachen, D.A. 2014. Cheatgrass invasion of sagebrush steppe: Impacts of vegetation structure on small mammals. M.Sc. Thesis. Bozeman, Montana: Montana State University. 96 p. Bess, J.A. 1997. The leafhopper species assemblages associated with native and replanted grasslands in southwest Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 94 p.
Bess, J.A. 1997. The leafhopper species assemblages associated with native and replanted grasslands in southwest Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 94 p. Bookman, P. 1983. Microsite utilization by Bromus tectorum L. and Poa pratensis L. in a meadow steppe community. Oecologia 56:413-418.
Bookman, P. 1983. Microsite utilization by Bromus tectorum L. and Poa pratensis L. in a meadow steppe community. Oecologia 56:413-418. Brey, C.W. 1998. Epidemiology of wheat curl mite (Aceria tosichella K.) and wheat streak mosaic virus on feral grass species and effect of glyphosate on wheat curl mite dispersal. Ph.D. Dissertation. Bozeman, MT: Montana State University. 136 p.
Brey, C.W. 1998. Epidemiology of wheat curl mite (Aceria tosichella K.) and wheat streak mosaic virus on feral grass species and effect of glyphosate on wheat curl mite dispersal. Ph.D. Dissertation. Bozeman, MT: Montana State University. 136 p. Britton, M. P. 1955. An ecological study of a relict grassland and an adjacent grazed pasture in Beaverhead Valley, Montana. M.S. thesis. Montana State University, Bozeman. 23 pp.
Britton, M. P. 1955. An ecological study of a relict grassland and an adjacent grazed pasture in Beaverhead Valley, Montana. M.S. thesis. Montana State University, Bozeman. 23 pp. Buckmaster, 2015. Community Composition Analysis of Altered Vegetation Communities Following the Release of Grazing Pressure. M.Sc. Thesis. Bozema, MT: Montana State University. 66 p.
Buckmaster, 2015. Community Composition Analysis of Altered Vegetation Communities Following the Release of Grazing Pressure. M.Sc. Thesis. Bozema, MT: Montana State University. 66 p. Burkhardt, A. 2019. Elucidating the effect of anthropogenic land management on soil nematode community structure. Ph.D. Dissertation. Bozeman, MT: Montana State University. 101 p.
Burkhardt, A. 2019. Elucidating the effect of anthropogenic land management on soil nematode community structure. Ph.D. Dissertation. Bozeman, MT: Montana State University. 101 p. Butler, M.A. 1996. The validity of using artificial nests to assess nest-predation rates in prairie nesting ducks. M.Sc. Thesis. Bozeman, MT: Montana State University. 82 p.
Butler, M.A. 1996. The validity of using artificial nests to assess nest-predation rates in prairie nesting ducks. M.Sc. Thesis. Bozeman, MT: Montana State University. 82 p. Carroll, M.W. 1998. Influence of a legume covercrop on volunteer wheat, the wheat curl mite, Aceria tosichella (K.) and wheat streak mosaic virus. M.Sc. Thesis. Bozeman, MT: Montana State University. 78 p.
Carroll, M.W. 1998. Influence of a legume covercrop on volunteer wheat, the wheat curl mite, Aceria tosichella (K.) and wheat streak mosaic virus. M.Sc. Thesis. Bozeman, MT: Montana State University. 78 p. Chadde, S.W. 1985. Initial recovery patterns of southwestern Montana foothill range. M.Sc. Thesis. Bozeman, MT: Montana State University. 103 p.
Chadde, S.W. 1985. Initial recovery patterns of southwestern Montana foothill range. M.Sc. Thesis. Bozeman, MT: Montana State University. 103 p. Corr, D.R. 1988. Effects of stress inducing factors on musk thistle (Carduus nutans L,) including--grass competition, Rhinocyllus conicus Froel., terminal flower loss, and insecticides. M.Sc. Thesis. Bozeman, MT: Montana State University. 86 p.
Corr, D.R. 1988. Effects of stress inducing factors on musk thistle (Carduus nutans L,) including--grass competition, Rhinocyllus conicus Froel., terminal flower loss, and insecticides. M.Sc. Thesis. Bozeman, MT: Montana State University. 86 p. Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp.
Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp. Dahal, D., S.P. Boyte, S. Parajuli, N.J. Pastick, M.J. Oimoen, and D. Shrestha. 2022. Early estimates of exotic annual grass (EAG) in the sagebrush biome, USA. U.S. Geological Survey data release, https://doi.org/10.5066/P9FVYOGD.
Dahal, D., S.P. Boyte, S. Parajuli, N.J. Pastick, M.J. Oimoen, and D. Shrestha. 2022. Early estimates of exotic annual grass (EAG) in the sagebrush biome, USA. U.S. Geological Survey data release, https://doi.org/10.5066/P9FVYOGD. Dillard, S.L. 2019. Restoring semi-arid lands with microtopography. M.Sc. Thesis. Bpzeman, MT: Montana State University. 97 p.
Dillard, S.L. 2019. Restoring semi-arid lands with microtopography. M.Sc. Thesis. Bpzeman, MT: Montana State University. 97 p. Douglass, R.J. 1973. Spatial interactions and microhabitat selections of two locally sympatric voles, Microtus montanus and Microtus pennsylvanicus. Ph.D. Dissertation. Bozeman, Montana: Montana State University. 48 p.
Douglass, R.J. 1973. Spatial interactions and microhabitat selections of two locally sympatric voles, Microtus montanus and Microtus pennsylvanicus. Ph.D. Dissertation. Bozeman, Montana: Montana State University. 48 p. DuBois, K.L. 1979. An inventory of the avifauna in the Long Pines of Southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 113 p.
DuBois, K.L. 1979. An inventory of the avifauna in the Long Pines of Southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 113 p. Egan, J.L. 1957. Some relationships between mule deer and alfalfa production in Powder River County, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 34 p.
Egan, J.L. 1957. Some relationships between mule deer and alfalfa production in Powder River County, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 34 p. Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p.
Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p. Eng, R.L. 1952. A two-summer study of the effects on bird populations of chlordane bait and aldrin spray as used for grasshopper control. M.Sc. Thesis. Bozeman, MT: Montana State University. 27 p.
Eng, R.L. 1952. A two-summer study of the effects on bird populations of chlordane bait and aldrin spray as used for grasshopper control. M.Sc. Thesis. Bozeman, MT: Montana State University. 27 p. Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p.
Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p. Fritzen, D.E. 1995. Ecology and behavior of Mule Deer on the Rosebud Coal Mine, Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 143 p.
Fritzen, D.E. 1995. Ecology and behavior of Mule Deer on the Rosebud Coal Mine, Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 143 p. Glazier, R.J. 1971. Ecological and morphological relationships of subspecies of Peromyscus maniculatus near St. Mary, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 41 p.
Glazier, R.J. 1971. Ecological and morphological relationships of subspecies of Peromyscus maniculatus near St. Mary, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 41 p. Gobeille, J.E. 1992. The effect of fire on Merriams turkey brood habitat in southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 61 p.
Gobeille, J.E. 1992. The effect of fire on Merriams turkey brood habitat in southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 61 p. Grove, A.J. 1998. Effects of Douglas fir establishment in southwestern Montana mountain big sagebrush communities. M. Sc.Thesis. Bozeman, MT: Montana State University. 150 p.
Grove, A.J. 1998. Effects of Douglas fir establishment in southwestern Montana mountain big sagebrush communities. M. Sc.Thesis. Bozeman, MT: Montana State University. 150 p. Guenther, G.E. 1989. Ecological relationships of bitterbrush communities on the Mount Haggin Wildlife Management Area. M.Sc. Thesis. Bozeman, MT: Montana State University. 73 p.
Guenther, G.E. 1989. Ecological relationships of bitterbrush communities on the Mount Haggin Wildlife Management Area. M.Sc. Thesis. Bozeman, MT: Montana State University. 73 p. Haile, K.F. 2011. Fuel load and heat effects on Northern mixed prairie and four prominent rangeland graminoids. M.Sc. Thesis. Bozeman, MT: Montana State University. 71 p.
Haile, K.F. 2011. Fuel load and heat effects on Northern mixed prairie and four prominent rangeland graminoids. M.Sc. Thesis. Bozeman, MT: Montana State University. 71 p. Harvey, A.J. 2019. Understanding the biology, ecology, and integrated management of Ventenata dubia. M.Sc. Thesis. Bozeman, MT: Montana State University. 117 p.
Harvey, A.J. 2019. Understanding the biology, ecology, and integrated management of Ventenata dubia. M.Sc. Thesis. Bozeman, MT: Montana State University. 117 p. Harvey, S.J. 1990. Responses of steppe plants to gradients of water soil texture and disturbance in Montana, U.S.A. Ph.D. Thesis. Bozeman, MT: Montana State University. 34 p.
Harvey, S.J. 1990. Responses of steppe plants to gradients of water soil texture and disturbance in Montana, U.S.A. Ph.D. Thesis. Bozeman, MT: Montana State University. 34 p. Hendricks, P., G.M. Kudray, S. Lenard, and B.A. Maxell. 2007. A Multi-Scale Analysis Linking Prairie Breeding Birds to Site and Landscape Factors Including USGS GAP Data. Helena, Mont: Montana Natural Heritage Program.
Hendricks, P., G.M. Kudray, S. Lenard, and B.A. Maxell. 2007. A Multi-Scale Analysis Linking Prairie Breeding Birds to Site and Landscape Factors Including USGS GAP Data. Helena, Mont: Montana Natural Heritage Program. Hodgson, J.R. 1970. Ecological distribution of Microtus montanus and Microtus pennsylvanicus in an area of geographic sympatry in southwestern Montana. Ph.D. Dissertation. Bozeman, Montana: Montana State University. 65 p.
Hodgson, J.R. 1970. Ecological distribution of Microtus montanus and Microtus pennsylvanicus in an area of geographic sympatry in southwestern Montana. Ph.D. Dissertation. Bozeman, Montana: Montana State University. 65 p. Hoffman, T.L. 1996. An ecological investigation of mountain big sagebrush in the Gardiner Basin. M.Sc. Thesis. Bozeman, MT: Montana State University. 84 p.
Hoffman, T.L. 1996. An ecological investigation of mountain big sagebrush in the Gardiner Basin. M.Sc. Thesis. Bozeman, MT: Montana State University. 84 p. Holeckek, J.L. 1976. Initial effects of different species treatments and fertilizer rates on a mine spoils rehabilitation. M.Sc. Thesis. Bozeman, MT: Montana State University. 91 p.
Holeckek, J.L. 1976. Initial effects of different species treatments and fertilizer rates on a mine spoils rehabilitation. M.Sc. Thesis. Bozeman, MT: Montana State University. 91 p. Husby, P.O. 1982. Effects of grazing on vegetation in the Artemisia tridentata-Festuca idahoensis habitat type. M.Sc. Thesis. Bozeman, MT: Montana State University. 76 p.
Husby, P.O. 1982. Effects of grazing on vegetation in the Artemisia tridentata-Festuca idahoensis habitat type. M.Sc. Thesis. Bozeman, MT: Montana State University. 76 p. Ito, D. 2011. Evaluation of susceptibility to wheat streak mosaic virus among small grains and alternative hosts in the great plains. M.Sc. Thesis. Bozeman, MT: Montana State University. 93 p.
Ito, D. 2011. Evaluation of susceptibility to wheat streak mosaic virus among small grains and alternative hosts in the great plains. M.Sc. Thesis. Bozeman, MT: Montana State University. 93 p. Johnson, J.D. 2004. Restoring native species to crested wheatgrass dominated rangelands. M.Sc.Thesis. Bozeman, MT: Montana State University. 58 p.
Johnson, J.D. 2004. Restoring native species to crested wheatgrass dominated rangelands. M.Sc.Thesis. Bozeman, MT: Montana State University. 58 p. Jorgensen, H.E. 1970. Ecological aspects of the life history of Agropyron smithii Rydb. in Central Montana, with related effects of selective herbicide treatments of rangeland. Ph.D. Dissertation. Bozeman, MT: Montana State University. 118 p.
Jorgensen, H.E. 1970. Ecological aspects of the life history of Agropyron smithii Rydb. in Central Montana, with related effects of selective herbicide treatments of rangeland. Ph.D. Dissertation. Bozeman, MT: Montana State University. 118 p. Kim, H.K. 1982. Epidemiological, genetical, and physiological studies of the bacterial leaf streak pathogen Xanthomonas campestris pv. translucens (J.J.R.) Dowson. Ph.D. Dissertation. Bozeman, MT: Montana State University. 83 p.
Kim, H.K. 1982. Epidemiological, genetical, and physiological studies of the bacterial leaf streak pathogen Xanthomonas campestris pv. translucens (J.J.R.) Dowson. Ph.D. Dissertation. Bozeman, MT: Montana State University. 83 p. Kisch, H.R. 2015. An inventory of carbon stocks under native vegetation and farm fields in south-central Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 67 p.
Kisch, H.R. 2015. An inventory of carbon stocks under native vegetation and farm fields in south-central Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 67 p. Kitchen, K.A. 2010. The influence of Douglas-fir and Rocky Mountain juniper on Wyoming and mountain big sagebrush cover in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 87 p.
Kitchen, K.A. 2010. The influence of Douglas-fir and Rocky Mountain juniper on Wyoming and mountain big sagebrush cover in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 87 p. Larson, C.D., E.A. Lehnhoff, and L.J. Rew. 2017. A warmer and drier climate in the northern sagebrush biome does not promote Cheatgrass invasion or change its response to fire. Oecologia 185:763-774.
Larson, C.D., E.A. Lehnhoff, and L.J. Rew. 2017. A warmer and drier climate in the northern sagebrush biome does not promote Cheatgrass invasion or change its response to fire. Oecologia 185:763-774. Larson, C.D., E.A. Lehnhoff, C. Noffsinger, and L.J. Rew. 2017. Competition between cheatgrass and bluebunch wheatgrass is altered by temperature, resource availability, and atmospheric CO2 concentration. Oecologia (2018) 186:855-868. https://doi.org/10.1007/s00442-017-4046-6
Larson, C.D., E.A. Lehnhoff, C. Noffsinger, and L.J. Rew. 2017. Competition between cheatgrass and bluebunch wheatgrass is altered by temperature, resource availability, and atmospheric CO2 concentration. Oecologia (2018) 186:855-868. https://doi.org/10.1007/s00442-017-4046-6 Law, D.J. 1999. A comparison of water table dynamics and soil texture under black cottonwood recent alluvial bar, beaked sedge, and Geyer's/Drummond's willow communities. M.Sc. Thesis. Bozeman, MT: Montana State University. 68 p.
Law, D.J. 1999. A comparison of water table dynamics and soil texture under black cottonwood recent alluvial bar, beaked sedge, and Geyer's/Drummond's willow communities. M.Sc. Thesis. Bozeman, MT: Montana State University. 68 p. Lehnhoff, E.A., L.J.Rew, J.M. Mangold, T. Seipel, and D. Ragen.2019. Integrated management of Cheatgrass (Bromus tectorum) with sheep grazing and herbicide. Agronomy 9, 315
Lehnhoff, E.A., L.J.Rew, J.M. Mangold, T. Seipel, and D. Ragen.2019. Integrated management of Cheatgrass (Bromus tectorum) with sheep grazing and herbicide. Agronomy 9, 315 Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p.
Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p. Matlock-Cooley, S.J. 1993. Interaction between Deermice, Antelope Bitterbrush, and cattle in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University 84 p.
Matlock-Cooley, S.J. 1993. Interaction between Deermice, Antelope Bitterbrush, and cattle in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University 84 p. Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p.
Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p. Morris, M.S. and J.E. Schwartz. 1957. Mule deer and elk food habits on the National Bison Range. Journal of Wildlife Management 21(2):189-193.
Morris, M.S. and J.E. Schwartz. 1957. Mule deer and elk food habits on the National Bison Range. Journal of Wildlife Management 21(2):189-193. Morton, M.A. 1976. Nutritional values of major mule deer winter forage species in the Bridger Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 105 p.
Morton, M.A. 1976. Nutritional values of major mule deer winter forage species in the Bridger Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 105 p. Mussgnug, G.L. 1972. The structure and performance of an adult population of Aulocara elliotti (Thomas) (Orthoptera, Acrididae) near Billings, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 97 p.
Mussgnug, G.L. 1972. The structure and performance of an adult population of Aulocara elliotti (Thomas) (Orthoptera, Acrididae) near Billings, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 97 p. Nyberg, H.E. 1980. Distribution, movements and habitat use of mule deer associated with the Brackett Creek winter range, Bridger Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 106 p.
Nyberg, H.E. 1980. Distribution, movements and habitat use of mule deer associated with the Brackett Creek winter range, Bridger Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 106 p. Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park. Ozeran, R.K. 2016. Disturbance and site characteristics relate to cheatgrass (Bromus tectorum) abundance on ranches in Montana foothills ecosystems. M.Sc. Thesis. Bozeman, MT: Montana State University. 88 p.
Ozeran, R.K. 2016. Disturbance and site characteristics relate to cheatgrass (Bromus tectorum) abundance on ranches in Montana foothills ecosystems. M.Sc. Thesis. Bozeman, MT: Montana State University. 88 p. Payson, E. 1996. Gap colonization in grasslands of the Northern Great Plains. M.Sc. Thesis. Bozeman, MT: Montana State University. 37 p.
Payson, E. 1996. Gap colonization in grasslands of the Northern Great Plains. M.Sc. Thesis. Bozeman, MT: Montana State University. 37 p. Plantenberg, P.L. 1983. Factors affecting vegetation development on mined land at Colstrip, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 121 p.
Plantenberg, P.L. 1983. Factors affecting vegetation development on mined land at Colstrip, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 121 p. Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p.
Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p. Rennick, R.B. 1981. Effects of prescribed burning on mixed prairie vegetation in southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 144 p.
Rennick, R.B. 1981. Effects of prescribed burning on mixed prairie vegetation in southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 144 p. Rens, E.N. 2003. Geographical analysis of the distribution and spread of invasive plants in the Gardiner Basin, MT. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p.
Rens, E.N. 2003. Geographical analysis of the distribution and spread of invasive plants in the Gardiner Basin, MT. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p. Rinella, M.J., A.D. Knudsen, J.S. Jacobs, and J.M. Mangold. 2020. Seeding causes long-term increases in grass forage production in invaded rangelands. Rangeland and Ecology Management 73(2020): 329-333.
Rinella, M.J., A.D. Knudsen, J.S. Jacobs, and J.M. Mangold. 2020. Seeding causes long-term increases in grass forage production in invaded rangelands. Rangeland and Ecology Management 73(2020): 329-333. Rundquist, V.M. 1973. Avian ecology on stock ponds in two vegetational types in north-central Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 112 p.
Rundquist, V.M. 1973. Avian ecology on stock ponds in two vegetational types in north-central Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 112 p. Sater, S. 2022. The insects of Sevenmile Creek, a pictorial guide to their diversity and ecology. Undergraduate Thesis. Helena, MT: Carroll College. 242 p.
Sater, S. 2022. The insects of Sevenmile Creek, a pictorial guide to their diversity and ecology. Undergraduate Thesis. Helena, MT: Carroll College. 242 p. Scow, K.L. 1981. Ecological distribution of small mammals at Sarpy Creek, Montana, with special consideration of the Deer Mouse, Peromyscus maniculatus. M.Sc. Thesis. Bozeman, Montana: Montana State University. 73 p.
Scow, K.L. 1981. Ecological distribution of small mammals at Sarpy Creek, Montana, with special consideration of the Deer Mouse, Peromyscus maniculatus. M.Sc. Thesis. Bozeman, Montana: Montana State University. 73 p. Seipel, T., L.J. Rew, K.T. Taylor, B.D. Maxwell, and E.A. Lehnhoff. 2018. Applied Vegetation Science 21:385-394.
Seipel, T., L.J. Rew, K.T. Taylor, B.D. Maxwell, and E.A. Lehnhoff. 2018. Applied Vegetation Science 21:385-394. Seipel, T.F. 2006. Plant species diversity in the sagebrush steppe of Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 87 p.
Seipel, T.F. 2006. Plant species diversity in the sagebrush steppe of Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 87 p. Skilbred, Chester L. 1979. Plant succession on five naturally revegetated strip-mined deposits at Colstrip, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 128 pp.
Skilbred, Chester L. 1979. Plant succession on five naturally revegetated strip-mined deposits at Colstrip, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 128 pp. Skinner, K.F. 1995. Plant and grasshopper community composition: indicators & interactions across three spatial scales. M.Sc. Thesis. Bozeman, MT: Montana State University. 144 p.
Skinner, K.F. 1995. Plant and grasshopper community composition: indicators & interactions across three spatial scales. M.Sc. Thesis. Bozeman, MT: Montana State University. 144 p. Sloane, Charles E. 2011. Effects of sugar beet pulp on cheatgrass and bluebunch wheatgrass growth under controlled conditions. M.Sc. Thesis. Bozeman, MT: Montana State University. 38 p.
Sloane, Charles E. 2011. Effects of sugar beet pulp on cheatgrass and bluebunch wheatgrass growth under controlled conditions. M.Sc. Thesis. Bozeman, MT: Montana State University. 38 p. Steerey, W. F. 1979. Distribution, range use and population characteristics of Mule Deer associated with the Schafer Creek winter range, Bridger Mountains, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 119 p.
Steerey, W. F. 1979. Distribution, range use and population characteristics of Mule Deer associated with the Schafer Creek winter range, Bridger Mountains, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 119 p. Stewart, S.T. 1975. Ecology of the West Rosebud and Stillwater bighorn sheep herds, Beartooth Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 130 p.
Stewart, S.T. 1975. Ecology of the West Rosebud and Stillwater bighorn sheep herds, Beartooth Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 130 p. Story, J.M. 1976. A study of Urophora affinis (Diptera: Tephritidae) released on spotted knapweed in Western Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 77 p.
Story, J.M. 1976. A study of Urophora affinis (Diptera: Tephritidae) released on spotted knapweed in Western Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 77 p. Taylor, K., T. Brummer, L.J. Rew, B.D. Maxwell. 2014. Bromus tectorum response to fire varies with climate conditions. Ecosystems 17(6): 960-973. http://dx.doi.org/10.1007/s10021-014-9771-7
Taylor, K., T. Brummer, L.J. Rew, B.D. Maxwell. 2014. Bromus tectorum response to fire varies with climate conditions. Ecosystems 17(6): 960-973. http://dx.doi.org/10.1007/s10021-014-9771-7 Thompson, W. L. 1993. Ecology of Merriam's Turkeys in relation to burned and logged areas in southeastern Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 195 p.
Thompson, W. L. 1993. Ecology of Merriam's Turkeys in relation to burned and logged areas in southeastern Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 195 p. Tschache, O.P. 1970. Effects of ecological changes induced by various sagebrush control techniques on small mammal populations. M.Sc. Thesis. Bozeman, MT: Montana State University. 51 p.
Tschache, O.P. 1970. Effects of ecological changes induced by various sagebrush control techniques on small mammal populations. M.Sc. Thesis. Bozeman, MT: Montana State University. 51 p. Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp.
Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp. Williams, K.L. 2012. Classification of the grasslands, shrublands, woodlands, forests and alpine vegetation associations of the Custer National Forest portion of the Beartooth Mountains in southcentral Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 376 p.
Williams, K.L. 2012. Classification of the grasslands, shrublands, woodlands, forests and alpine vegetation associations of the Custer National Forest portion of the Beartooth Mountains in southcentral Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 376 p. Wiman, N.G. 2001. Dynamics of leafy spurge (Euphorbia esula L.) infested plant communities influenced by flea beetles in the Aphthona complex (Colepotera: Chrysomelidae). M.Sc. Thesis. Bozeman, MT: Montana State University. 148 p.
Wiman, N.G. 2001. Dynamics of leafy spurge (Euphorbia esula L.) infested plant communities influenced by flea beetles in the Aphthona complex (Colepotera: Chrysomelidae). M.Sc. Thesis. Bozeman, MT: Montana State University. 148 p. Wood, A.K. 1987. Ecology of a prairie mule deer population. Ph.D. Dissertation. Bozeman, MT: Montana State University. 205 p.
Wood, A.K. 1987. Ecology of a prairie mule deer population. Ph.D. Dissertation. Bozeman, MT: Montana State University. 205 p.
- Web Search Engines for Articles on "Cheatgrass"





