View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Common St. John's-wort - Hypericum perforatum
Other Names:
Klamath Weed, Common St. Johnswort, St. Johnswort
State Rank Reason (see State Rank above)
Hypericum perforatum is native to portions of Europe, North Africa, and Asia and was introduced into North America (Piper in Sheley and Petroff 1999). A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
General Description
PLANTS: Multi-stemmed, herbaceous perennial that has a taproot and is rhizomatous. Stems grow from 25 to 75 cm tall. Flowering stems are smooth, reddish, and upright with two opposite longitudinal ridges and black glands. Stems are somewhat woody at the base and branched near their tops. From the root crown also grow leafy non-flowering stems. Sources: Piper in Sheley and Petroff 1999; Lesica et al. 2012.
LEAVES: Leaves are sessile and arranged opposite. Leaf blades are dark green above, light green below, lanceolate in shape, 1–3 cm long, and dotted with translucent glands. Hold leaves to the light or use a hand-lens to see the translucent glands. Leaf margins have black glands, are smooth (entire), and revolute. Sources: Piper in Sheley and Petroff 1999; Lesica et al. 2012.
INFLORESCENCE: Yellow flowers are arranged in an open, flat-topped cyme. Each flower has 5 green sepals that are linear-lanceolate in shape and 5–8 mm long. Each flower has 5 golden-yellow petals, 8–14 mm long, and edged with black glands. Stamens are numerous and noticeable. Sources: Piper in Sheley and Petroff 1999; Lesica et al. 2012.
The specific epithet of perforatum refers to the translucent glands that appear to put holes in the leaf.
Phenology
Plants emerge in spring and may re-grow in fall with enough soil moisture. Flowering occurs June through September. Seeds develop from mid-summer to late fall. Source: Piper in Sheley and Petroff 1999.
Diagnostic Characteristics
Montana has 3 native and 1 exotic species of
Hypericum. All have leaves that are opposite, sessile, entire, and dotted with translucent glands. Hold a leaf up to the sunlight and see the glands.
Common St. John’s-wort -
Hypericum perforatum, exotic and noxious
Distinguished by:
* Size: Mature plants are 25-75 cm tall.
* Flowers: 5-yellow petals outlined by black glands and showy yellow stamens.
* Sepal: 5 linear-lanceolate with acute tips.
* Branches: reddish-colored that help make the plant more noticeable in fall.
* Fruit: a 3-valved capsules with many, tiny seeds.
* Roots: taprooted and rhizomatous.
Tinker’s-penny -
Hypericum anagalloides, native and desirable
* Mature plants are 2-12 cm tall, grow along the ground, and root at leaf nodes.
* Smaller flowers with 5-yellow petals not outlined by black glands and slightly longer than sepals.
* Habitat of wetlands and wet soil in meadows along streams in montane and subalpine zones.
Larger Canadian St. John’s-wort -
Hypericum majus, native and desirable
* Mature plants are 5-25 cm tall, grow decumbant.
* Smaller flowers with 5-yellow petals not outlined by black glands and about as long as sepals.
* Habitat along rivers, ponds, and lakes in valley and montane zones.
Western St. John’s-wort –
Hypericum scouleri, native and desirable
* Mature plants are 5-60 cm tall.
* Large flowers with 5-yellow petals outlined by black glands.
* Sepals are ovate with rounded tips and amber veins.
* Rhizomatous and stoloniferous.
* Habitat is moist to wet areas in meadows, along streambanks, and on ledges from the valleys to the alpine zones.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Common St. John’s-wort is native to portions of Europe, North Africa, northern India, China, and Japan (Piper in Sheley and Petroff 1999). It has been introduced into numerous countries because of its medicinal and ornamental uses. In the U.S. it was first reported in Pennsylvania either in 1696 (unknown source in Mangold, Sheley, and Brown 2017) or in 1793 (Sampson and Parker 1930) and carried to Oregon between 1840 and 1850 (Campbell and Delfosse 1984). Herbarium specimens posted on the Consortium of Pacific Northwest Herbaria (CPNWH) portal document its presence in Oregon in 1877, British Columbia in 1895, Washington in 1908, Idaho in 1921, and Montana in 1934 (Posted as of January 28, 2019 at http://www.pnwherbaria.org). The earliest Montana specimens document it from Flathead County in 1934, Mineral County in 1935, Sanders County in 1936, and Lake County in 1937 (Posted as of January 28, 2019 at http://www.pnwherbaria.org).
The future distribution of invasive weed species across the network of major roads in Montana was modeled (Adhikari et al. 2020). The model projected that by 2040 habitat suitability along roadsides would expand for 7 of the 11 Montana noxious species. In comparison to today's distribution the model predicted that suitable habitat for Hypericum perforatum would increase the most, expanding by six-folds.
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
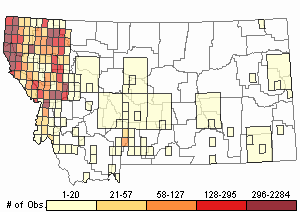
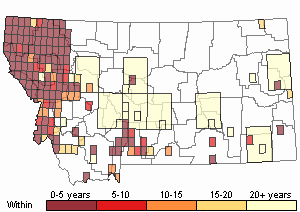
Observations in Montana Natural Heritage Program Database
Number of Observations: 19602
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
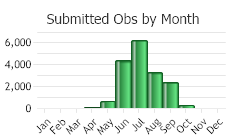
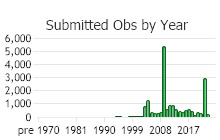
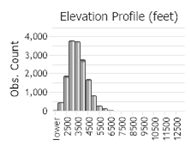 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Common St. John’s-wort grows in grasslands, open forest, clearings in forested areas, pastures, and along roadsides in valleys and montane zones in Montana (Lesica et al. 2012). Elsewhere it can be a problem in orchards and in fruit and conifer plantations (Piper in Sheley and Petroff 1999).
Ecology
The plant colonizes disturbed and apparently pristine rangelands in the western U.S. Common St. John’s-wort prefers south and southeast-facing slopes and well-drained gravelly or sandy soils, but will grow in heavier soils (Piper
in Sheley and Petroff 1999). It does not grow well in soils that are perennially moist or where heavily shaded (Piper
in Sheley and Petroff 1999).
Common St. John’s-wort is toxic to Common St. John’s-wort is toxic to cattle, horses, sheep, and goats [see Threats or Limiting Factors] (Piper
in Sheley and Petroff 1999; Mangold, Sheley, and Brown 2017).
CULTURALSt. John’s-wort plants make an oil infusion that in its finished state is clear and ruby red and can be added to salves and ointments for topical application or other uses. Its medicinal functions are as an astringent (tightens tissues; used to check bleeding, diarrhea, etc.), anti-inflammatory, vulnerary (heals internal or external wounds), and sedative (calms the nerves) (Tilford 1993).
The Cherokee people used Common St. John’s-wort for many medicinal needs including: to stop menstruation; for bowel complaints; when crushed and sniffed to stop nosebleeds; as a milky substance it was rubbed on sores; used for venereal disease; used in a wash to give babies strength; as a poultice for snake bites (Moerman 1986).
The Iroquois people used Common St. John’s-wort for fevers and to prevent sterility (Moerman 1986).
The Montagnais people used Common St. John’s-wort to prepare a cough medicine (Moerman 1986).
POLLINATORS The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus vagans,
Bombus auricomus,
Bombus fervidus,
Bombus rufocinctus,
Bombus ternarius,
Bombus terricola,
Bombus pensylvanicus,
Bombus bimaculatus,
Bombus griseocollis, and
Bombus impatiens (Heinrich 1976, Thorp et al. 1983, Colla and Dumesh 2010, Colla et al. 2011, Williams et al. 2014).
Reproductive Characteristics
FRUIT [Adapted from Piper in Sheley and Petroff 1999]
Fruit is a capsule, egg-shaped, 3-valved, and 5–8 mm long. At maturity the capsule bursts open to expose numerous seeds. Seeds are somewhat cylindrical, pointed at the ends, coarsely pitted, and shiny brown.
ROOTS [Adapted from Piper in Sheley and Petroff 1999]
The taproot is stout and penetrate the soil to a depth of 5 feet. Rhizomes grow 2-3 inches below the soil surface, horizontally, and can extend 3 feet. On the rhizomes vegetative buds sprout into new root crowns.
LIFE CYCLE [Adapted from Piper in Sheley and Petroff 1999]
Seeds germinate in summer and seedlings may take several years to mature. When seedlings reach the 2- to 4-leaf stage, the leaves are sessile, rounded, and have smooth edges. At the 6-leaf stage, black glands are evident on their leaves. Seedlings compete poorly with adjacent plants for light, nutrients, space, and moisture. Seedling mortality can be high.
Neighboring mature plants may come from the same or different woody root crowns from which a vertical root (taproot) and horizontal roots (rhizomes) grow. Established root crows can may produce up to 30 flowering stems each year. Root crown density has been found to range from 12 to 37 per square meter. Buds on the rhizomes produce new crowns, mostly in the spring and fall. Connecting rhizomes can decay which causes these root crowns to become independent plants.
Most mature plants (2 years or older) re-grow stems in spring and produce floral shoots by June. Flowering can occur through September but is dependent upon moisture. By mid-summer most flowering has ceased and the developing seed capsules are moist, green, and sticky. By mid- to late-summer stems turn reddish-brown and dieback, but fall re-growth can occur if soil moisture increases. Stems that re-grow in the fall are spindly and don’t flower and persist until temperatures become too cold. Dead stems can remain attached to the root crown for a year or more. By late fall, capsules can release 400-500 ripe seeds. It has been estimated that an average, single plant can produce 15,000 to 33,000 seeds each year.
Seeds are dispersed by wind and adhere to passing animals and humans. The gelatinous seed coat makes them sticky. Animals that ingest the capsules can disperse them in their feces. Seeds can also be transported by water, vehicles and other mechanical equipment, mud adhered to boots, and other manmade methods. Seeds require 4-6 weeks of time to ripen before they can germinate. Seeds that are buried at 5mm or deeper will not germinate.
Management
See
Noxious Weed Education VideoOnce plants become established Common St. John’s-wort is very difficult to control or remove.
An integrated vegetative management approach provides the best long-term control, and requires that land-use objectives and a desired plant community be identified (Shelly et al.
in Sheley and Petroff 1999). Once identified an integrated weed management strategy that promotes a weed-resistant plant community and serves other land-use objectives such as livestock forage, wildlife habitat, or recreation can be developed.
PREVENTION [Adapted from Piper
in Sheley and Petroff 1999]
Preventing the establishment of Common St. John’s-wort can be accomplished by many practices:
* Learn how to accurately identify Common St. John’s-wort in order to detect occurrences and know where to implement control methods.
* Prevent vehicles from driving through and animals from grazing within infested areas.
* Thoroughly wash the undercarriage of vehicles and wheels in a designated area before moving to uninfested areas.
* Frequently monitor for new plants, and when found implement effective control methods.
* Maintain proper grazing management that creates resilience to noxious weed invasion.
* Do not pick the flowers or transport plants. Where possible, contribute to or develop educational campaigns to help eradicate or reduce Common St. John’s-wort populations.
PHYSICAL and CULTURAL CONTROLS [Adapted from Piper
in Sheley and Petroff 1999]
Hand-pulling is effective for young populations or isolated plants. Rhizomes that remain in the soil can sprout new plants. Populations with a seedbed or multiple ages will be difficult to control with only hand-pulling. Hand-pulling plants in combination with other control methods will likely be more successful at removing the population.
Repeated Tilling in intensively cropped situations is effective.
Revegetating land with competitive, locally adapted, and palatable grasses, legumes, or other desirable forbs will develop a plant community that is more resilient to Common St. John’s-wort. A program in Australia found that cultivation, fertilization, and re-seeding with a competitive perennial grass controlled Common St. John’s-wort over a 2 to 5 year time period. Plants do poorly in shaded environments. Sites that are re-forested can reduce populations, but will not completely eradicate plants. Revegetation in combination with other control methods can be more effective to eradicate or reduce this plant.
Prescribed Burning is not an effective control method. Burning stimulates seed germination and re-growth from buds on the root crowns and rhizomes. Repeated burning diminishes decaying organic matter and deletes nutrients, creating conditions that favor its re-establishment.
Mowing is not an effective control method because established populations have multiple aged plants and flowering/seeding can occur in spring, summer, and/or fall. Mowing can reduce flowering and seed formation, but would need to be repeated during the growing season and in subsequent years. Mowing encourages the sprawling growth of stems from buds on the root crowns and rhizomes.
CHEMICAL CONTROLS [Adapted from Piper
in Sheley and Petroff 1999]
Herbicides can be effective, especially when properly integrated with intensive pasture management. The herbicide type and concentration, application time and method, environmental constraints, land use practices, local regulations, and other factors will determine its effectiveness and impact to non-target species. Strict adherence to application requirements defined on the herbicide label will reduce risks to human and environmental health. Consult your County Extension Agent and/or Weed District for information on herbicidal control. Chemical information is also available at
Greenbook.
2,4-D (2 pounds acid equivalent per acre (ae/ac)) applied to foliage will kill plants in the seedling and pre-flowering stages in non-cropland sites.
Picloram (0.125 to 1.50 pounds per active ingredient per acre (ae/ac)) or
glyphosate (0.187 to 0.375 ae/ac applied in the spring can suppress plants in pastures, rangelands, and other non-cropland sites.
Mesulfuron (0.04 pounds ai/ac) applied after plant emergence can be an effective control.
BIOLOGICAL CONTROLS The first attempt in North America to control an exotic plant by the intentional introduction of insects was done to control Common St. John’s-wort in California in the 1940’s (Piper
in Sheley and Petroff 1999). Inspiration came from Australia where European insects were released into Australia in the 1940s and successfully suppressed Common St. John’s-wort (Piper
in Sheley and Petroff 1999).
St. John's-wort Beetle (
Chrysolina hypericin) and
Klamath Weed Beetle (
Chrysolina quadrigemina) feed on foliage of Common St. John’s-wort during their larval stage. This reduces the plant’s ability to store nutrients in their roots, which can lessen winter survival (https://integratedweedcontrol.com). These insects have significantly reduced populations in the Pacific Northwest states (https://integratedweedcontrol.com). They are best suited to sunny and warm sites in mountainous areas (Mangold, Sheley, and Brown 2017).
Chrysolina hypericin performs better where sites are wetter.
St. John’s-wort Inchworm (
Aplocera plagiata) was introduced into the Pacific Northwest. Larvae of this moth feed on leaves and flowers. When numerous enough the plants are defoliated, and flower and seed formation are inhibited (https://integratedweedcontrol.com). This insect lives best in dry, open areas with soils that are sandy, rocky, or of limestone parent material (Mangold, Sheley, and Brown 2017). Insects don’t thrive where precipitation is high.
St. John’s-wort Root Borer (
Agrilus hyperici) lives best in dry, mountainous areas (Mangold, Sheley, and Brown 2017).
St. John’s-wort Midge (
Zeuxidiplosis giardi) prefers damp sites and does not do well in dry, wind-prone, or heavily grazed areas(Mangold, Sheley, and Brown 2017). Thus, it has not established well in Montana (Mangold, Sheley, and Brown 2017).
GRAZING CONTROLS Common St. John’s-wort is toxic to Common St. John’s-wort is toxic to cattle, horses, sheep, and goats [see Threats or Limiting Factors] (Piper
in Sheley and Petroff 1999; Mangold, Sheley, and Brown 2017). However, grazing management that maintains the viability of dense, desirable forage will be more resilient to invasion by Common St. John’s-wort.
Contact information for local county Weed District Coordinators can be found on the
Montana Weed Control Association Contacts WebpageUseful Links:Montana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionIntegrated Noxious Weed Management after WildfiresStewardship Responsibility
Threats or Limiting Factors
Common St. John’s-wort forms dense stands and displaces native and exotic forage plant species, reducing livestock and wildlife carrying capacities. The dense stands decrease plant species diversity.
Common St. John’s-wort can be toxic to cattle, horses, sheep, and goats if ingested in sufficient quantity (Piper in Sheley and Petroff 1999). The minimum toxic dose of foliage for cattle and sheep is about 1% and 4% of their body weight, respectively. Goats tend to be more resistant.
The chemical, hypericin is produced from glands on the stems, leaves, flower, stamens, and fruits (Piper in Sheley and Petroff 1999). The toxicity causes severe dermatitis (blistering and itching) that arises when animals ingest the plant when exposed to intense sunlight (Piper in Sheley and Petroff 1999). The phototoxic substance, hypericin is unaltered by ingestion or digestion, is not removed by the liver, and is absorbed into the bloodstream. Fresh and dried plants of all growth stages are toxic, but the effects are greatest from flowering plants. Symptoms become detectable 2 to 21 days after ingestion. Animals can recover if they stop eating Common St. John’s-wort. Death usually does not occur directly from eating the plant, but indirectly from other factors, such as, not drinking water.
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Adhikari, A., L.J. Rew, K.P. Mainali, S. Adhikari, and B.D. Maxwell. 2020. Future distribution of invasive weed species across the major road network in the state of Montana, USA. Regional Environmental Change 20(60):1-14. https://doi.org/10.1007/s10113-020-01647-0
Adhikari, A., L.J. Rew, K.P. Mainali, S. Adhikari, and B.D. Maxwell. 2020. Future distribution of invasive weed species across the major road network in the state of Montana, USA. Regional Environmental Change 20(60):1-14. https://doi.org/10.1007/s10113-020-01647-0 Campbell, M.H. and E.S. Delfosse. 1984. The Biology of Australian Weeds. 13. Hypericum perforatum J. Aust. Inst. Agric. Sci. 50:63-73.
Campbell, M.H. and E.S. Delfosse. 1984. The Biology of Australian Weeds. 13. Hypericum perforatum J. Aust. Inst. Agric. Sci. 50:63-73. Colla, S., L. Richardson, and P. Williams. 2011. Bumble bees of the eastern United States. Washington, DC: USDA Forest Service, Pollinator Partnership. 103 p.
Colla, S., L. Richardson, and P. Williams. 2011. Bumble bees of the eastern United States. Washington, DC: USDA Forest Service, Pollinator Partnership. 103 p. Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Mangold, Jane, Roger Sheley, and Melissa Brown. 2017. St. Johnswort: Identification, Biology, and Integrated Management. MontGuide MT199810AG Revised June 2017. Montana State University Extension, Bozeman, Montana
Mangold, Jane, Roger Sheley, and Melissa Brown. 2017. St. Johnswort: Identification, Biology, and Integrated Management. MontGuide MT199810AG Revised June 2017. Montana State University Extension, Bozeman, Montana Moerman, Daniel E. 1986. Medicinal Plants of Native America. Research Reports in Ethnobotany, Contribution 2. Technical Reports, Number 19, Volumes 1 and 2. University of Michigan Museum of Anthropology, Ann Arbor, Michigan.
Moerman, Daniel E. 1986. Medicinal Plants of Native America. Research Reports in Ethnobotany, Contribution 2. Technical Reports, Number 19, Volumes 1 and 2. University of Michigan Museum of Anthropology, Ann Arbor, Michigan. Sampson, A.W., and K.W. Parker. 1930. St Johns-wort on Rangelands of California. Calif. Agric. Exp. Sta. Bull. 503.
Sampson, A.W., and K.W. Parker. 1930. St Johns-wort on Rangelands of California. Calif. Agric. Exp. Sta. Bull. 503. Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon. Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79.
Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79. Tilford, Gregory L. 1993. The EcoHerbalist's Fieldbook-Wildcrafting in the Mountain West. Mountain Weed Publishing, Conner, Montana.
Tilford, Gregory L. 1993. The EcoHerbalist's Fieldbook-Wildcrafting in the Mountain West. Mountain Weed Publishing, Conner, Montana. Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America. Princeton, NJ: Princeton University Press. 208 p.
Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America. Princeton, NJ: Princeton University Press. 208 p.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Cope, M.G. 1992. Distribution, habitat selection and survival of transplanted Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus) in the Tobacco Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 60 p.
Cope, M.G. 1992. Distribution, habitat selection and survival of transplanted Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus) in the Tobacco Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 60 p. Dillard, S.L. 2019. Restoring semi-arid lands with microtopography. M.Sc. Thesis. Bpzeman, MT: Montana State University. 97 p.
Dillard, S.L. 2019. Restoring semi-arid lands with microtopography. M.Sc. Thesis. Bpzeman, MT: Montana State University. 97 p. Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p.
Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p. Johnson, T. W. 1982. An analysis of pack and saddle stock grazing areas in the Bob Marshall Wilderness. M.Sc.Thesis. Bozeman, MT: Montana State University. 105 p.
Johnson, T. W. 1982. An analysis of pack and saddle stock grazing areas in the Bob Marshall Wilderness. M.Sc.Thesis. Bozeman, MT: Montana State University. 105 p. Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Morris, M. S., and J. Meuchel. 1956. The problem of St. Johnswort: a noxious plant in western Montana. Montana For. And Cons. Exp. Sta., School of For., University of Montana, Missoula. Bull. No.4. 8 pp.
Morris, M. S., and J. Meuchel. 1956. The problem of St. Johnswort: a noxious plant in western Montana. Montana For. And Cons. Exp. Sta., School of For., University of Montana, Missoula. Bull. No.4. 8 pp. Nelson, H.S. 1955. The phenology of goatweed, Hypericum perforatum in relation to the behavior of goatweed beetles, Chrysolina gemellata and C. hyperici, in western Montana. M.S. thesis. Montana State University, Missoula. 45 pp.
Nelson, H.S. 1955. The phenology of goatweed, Hypericum perforatum in relation to the behavior of goatweed beetles, Chrysolina gemellata and C. hyperici, in western Montana. M.S. thesis. Montana State University, Missoula. 45 pp.
- Web Search Engines for Articles on "Common St. John's-wort"





